Recalls
Latest FDA recalls, withdrawals, and safety alerts.
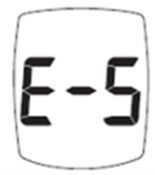
FDA Recall: TRUE METRIX Blood Glucose Monitoring Systems
As currently written, the Owner’s Booklets/System Instructions for Use fails to emphasize that users must seek medical attention immediately if they …

FDA Recall: No Brand Dried Croaker Fish
Product was not adequately eviscerated and may harbor harmful bacteria or toxins

FDA Recall: Why Not Natural Organic Moringa – Green for Potential Foodborne Illness - Salmonella
Potential Foodborne Illness - Salmonella

FDA Recall: Neogen Vet HYCOAT® Hyaluronate Sodium for Micorbial Contamination
Micorbial Contamination

FDA Recall: Fu Zhou Fish balls for Undeclared Allergen – Sesame, Wheat
Undeclared Allergen – Sesame, Wheat

FDA Recall: Navitas Organice Organic Chia Seeds for Potential Foodborne Illness - Salmonella
Potential Foodborne Illness - Salmonella

FDA Recall: IKM Metal Cookware Items for Potential Foodborne Illness – Lead contamination
Potential Foodborne Illness – Lead contamination

FDA Recall: Genova Yellowfin Tuna for Potential contamination with Clostridium botulinum
Potential contamination with Clostridium botulinum

FDA Recall: FreeStyle Libre FreeStyle Libre 3 and
Internal testing determined that some sensors may provide incorrect low glucose readings.

FDA Recall: Live it Up Super Greens Original and for Potential Foodborne Illness - Salmonella
Potential Foodborne Illness - Salmonella

FDA Recall: Spring & Mulberry Multiple Flavors of Chocolate for Potential Foodborne Illness - Salmo…
Potential Foodborne Illness - Salmonella

FDA Recall: Outside the Breadbox Bread Crumbs for Potential or Undeclared Allergen – Egg and Milk
Potential or Undeclared Allergen – Egg and Milk

FDA Recall: Spring & Mulberry Mint Leaf Date Sweetened for Potential Foodborne Illness - Salmonella
Potential Foodborne Illness - Salmonella

FDA Recall: Karison Panjiri, pinni, and laddoo for Potential or Undeclared Allergen – Milk
Potential or Undeclared Allergen – Milk

FDA Recall: Modern Warrior Dietary supplement capsules marketed for Unapproved new drug found to co…
Unapproved new drug found to contain undeclared 1,4-DMAA and aniracetam, and tianeptine

