FDA Recall: Vetoquinol Folltropin Injectable Kits for Cattle Pulled Due to Particulate Contamination
Quick take: Vetoquinol USA has issued a nationwide recall of six lots of Folltropin® injectable kits for cattle after visible particulates were discovered in the accompanying sterile diluent vials. Quarantine the affected kits immediately and follow the return instructions below.
Why Vetoquinol Folltropin Injectable Kits Were Recalled
During a routine inspection of retained samples, Vetoquinol detected visible particulate matter in the sterile bacteriostatic saline diluent packaged with certain Folltropin® injectable kits. Injecting products that contain particulate contamination can cause local or systemic reactions in cattle, including:
- Pain, swelling, or lesions at the injection site
- Fever, labored breathing, or other hypersensitivity signs
- General decline in activity or appetite
Although no adverse events had been reported to Vetoquinol or the U.S. Food and Drug Administration (FDA) at the time of the announcement, the company initiated the voluntary recall out of an abundance of caution.
Vetoquinol Folltropin Recall: Affected Lot Numbers and Key Details
- Product: Folltropin (porcine pituitary–derived follicle-stimulating hormone for injection)
- Presentation: Dual-vial kit (20 mL lyophilized powder + 20 mL sterile saline diluent)
- Distribution: Through U.S. veterinary distributors nationwide
- Recall scope: Six kit lots distributed between Aug 30 2023 and Oct 28 2025
| Kit Lot # | Diluent Lot # | Expiration | Distribution Begin | Distribution End |
|---|---|---|---|---|
| 510578 | 840915 | 05/31/2026 | 08/30/2023 | 10/23/2023 |
| 510579 | 844585 | 05/31/2026 | 10/23/2024 | 11/06/2024 |
| 510580 | 844583 | 05/31/2026 | 11/06/2024 | 06/18/2025 |
| 510581 | 934975 | 01/31/2027 | 06/18/2025 | 12/13/2025 |
| 717059 | 934976 | 01/31/2027 | 06/09/2025 | 10/28/2025 |
| 510582 | 934973 | 01/31/2027 | 12/13/2025 | 06/09/2026 |
Sources
Action Steps for Cattle Producers and Veterinarians
- Quarantine any Folltropin kits bearing the lot numbers listed above.
- Discontinue use of the recalled kits immediately. The Folltropin Dual Pack (powder-only) is not included in this recall.
- Contact Vetoquinol USA Customer Service at 1-800-267-5707 (Mon–Fri, 8 a.m.–5 p.m. CST) or email customerserviceusa@vetoquinol.com for return or disposal instructions.
- Report any adverse animal reactions to your veterinarian and to the FDA at 1-888-FDA-VETS or online at fda.gov/reportanimalae.
FDA Recall Images of Vetoquinol Folltropin Kits
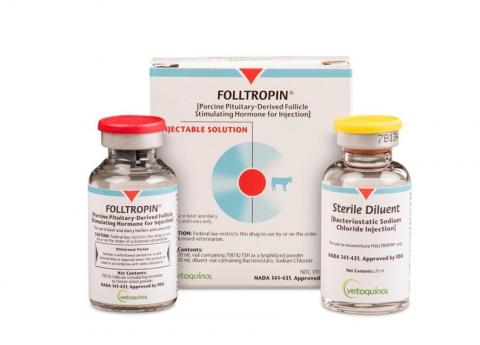
Stay Informed on the Vetoquinol Folltropin Recall
The FDA posted the recall notice on December 2, 2025. Review the full announcement (linked above) for ongoing updates and additional guidance.
Frequently Asked Questions
1. What Vetoquinol Folltropin product is being recalled?
Six lots of the Vetoquinol Folltropin injectable kit—containing FSH powder and a separate 20 mL sterile diluent vial—are being recalled. Lot numbers are listed earlier in this article.
2. Why did Vetoquinol recall these Folltropin kits?
Routine quality-control testing identified particulate matter in the diluent vial. Injecting contaminated diluent poses a risk of injection-site inflammation and systemic hypersensitivity in cattle.
3. Has the FDA received injury reports tied to Vetoquinol Folltropin?
No injuries or adverse events related to the recalled Vetoquinol Folltropin lots had been reported to either the company or the FDA at the time of the recall announcement.
4. Is the Vetoquinol Folltropin Dual Pack affected?
No. The Dual Pack contains only lyophilized Folltropin powder and does not include the potentially contaminated diluent vial.
5. How do I return or dispose of the recalled Vetoquinol Folltropin kits?
First quarantine the affected lots, then call Vetoquinol USA at 1-800-267-5707 or email customerserviceusa@vetoquinol.com for prepaid return shipping labels or approved disposal instructions.
6. Where can I read the full FDA notice on the Vetoquinol Folltropin recall?
The complete notice is published on the FDA website; see the Sources section above for a direct link.
7. Is there an alternative to Vetoquinol Folltropin while the recall is in effect?
Discuss replacement follicle-stimulating hormone products with your herd veterinarian. Do not substitute recalled Vetoquinol Folltropin kits with compounded or unapproved products without veterinary guidance.