FDA Recall: TRUE METRIX Blood Glucose Meters – E-5 Labeling Correction
Immediate action: Trividia Health has issued a labeling correction for every TRUE METRIX blood-glucose meter after discovering that the E-5 error message may downplay the need for urgent care. If you see an E-5 code while experiencing symptoms of very high blood sugar, follow the new instructions below and contact a healthcare professional right away.
Why the TRUE METRIX labeling correction matters
- Primary hazard: Earlier manuals failed to direct users to seek emergency treatment when the E-5 error code appears alongside signs of severe hyperglycemia (blood glucose > 600 mg/dL).
- Reported injuries: According to the FDA, 114 serious injuries and one death have been linked to delayed care since the product’s 2014 launch.
- Potential outcome: Untreated hyperglycemia can lead to diabetic ketoacidosis, coma, or death.
Which TRUE METRIX models are affected?
All TRUE METRIX-branded meters sold in the United States, United Kingdom, Mexico, Australia, and the Caribbean are included, as are store-brand versions distributed through pharmacies and medical suppliers.
- TRUE METRIX
- TRUE METRIX AIR
- TRUE METRIX GO
- TRUE METRIX PRO
Updated E-5 error instructions for TRUE METRIX meters
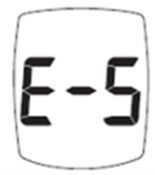
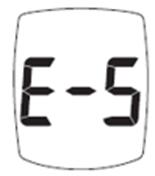
The E-5 message may indicate either a strip problem or a dangerously high glucose level. Follow these FDA-approved steps:
- Retest with a fresh test strip.
- If the error repeats and you have symptoms—fatigue, extreme thirst, frequent urination, or blurry vision—seek emergency medical care immediately.
- If you have no symptoms, test again. If E-5 persists, call Trividia Health at 1-800-803-6025 (Mon–Fri, 8 a.m.–8 p.m. EST).
Should you return or replace your TRUE METRIX meter?
No. The hardware is safe; only the instruction manuals require updating. You may keep using your device once you understand the revised E-5 guidance.
What TRUE METRIX users and retailers should do now
- Display the official Product Notice wherever meters are stored or sold.
- Share the notice with current TRUE METRIX customers whenever possible.
- Download updated manuals at trividiahealth.com/E-5productnotice.
- Report adverse events to FDA MedWatch online or via 1-800-332-1088.
Official recall images
Frequently Asked Questions
1. Why is there an FDA recall for TRUE METRIX blood glucose meters?
The E-5 instructions in earlier manuals did not emphasize that users showing hyperglycemia symptoms need emergency care, increasing the risk of life-threatening delays.
2. Do I need to stop using my TRUE METRIX meter immediately?
No. Continue testing but follow the revised E-5 steps and monitor yourself for signs of high blood sugar.
3. Which TRUE METRIX models are part of this recall?
Every TRUE METRIX, TRUE METRIX AIR, TRUE METRIX GO, and TRUE METRIX PRO meter—plus private-label versions—is covered by the labeling correction.
4. Will Trividia Health replace my TRUE METRIX instruction manual?
Yes. Updated manuals are free to download, and printed notices are being distributed to pharmacies, clinics, and retailers.
5. How do I report an adverse event related to my TRUE METRIX meter?
File a MedWatch report at fda.gov/medwatch/report.htm or request FDA Form 3500 by calling 1-800-332-1088.
6. What hyperglycemia symptoms should I watch for when using a TRUE METRIX meter?
Common warning signs include extreme thirst, frequent urination, fatigue, blurred vision, nausea, and fruity-smelling breath. If these occur with an E-5 error, seek medical help immediately.