ReBoost Nasal Spray Recall: FDA Warns of Microbial Contamination in Lot 224268
MediNatura New Mexico, Inc. has issued a voluntary nationwide recall of one lot of ReBoost Nasal Spray after routine quality testing detected yeast, mold and Achromobacter bacteria. Consumers—especially those who are immunocompromised—should locate lot 224268, discontinue use immediately, and follow the refund or return instructions below.
ReBoost Nasal Spray Recall — Quick Facts
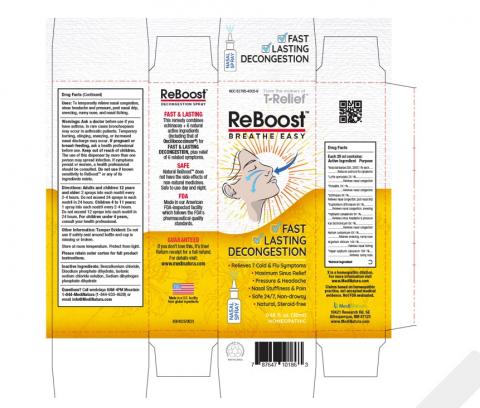
- Product: ReBoost Nasal Spray, 0.68 fl oz (20 mL) bottle in white/yellow carton
- Affected Lot / Expiry: 224268 — expires 12/2027
- Identifiers: NDC 62795-4005-9 • UPC 787647101863
- Reason for Recall: Yeast, mold, and Achromobacter contamination above specifications
- Distribution: Nationwide retail stores & medinatura.com
- Immediate Action: Stop use; obtain refund or replacement
- Consumer Contact: 800-621-7644 • recall@medinatura.com
Sources
Why the ReBoost Nasal Spray Recall Matters
Spraying a contaminated solution into the nasal passages can introduce harmful microbes directly into the sinuses and lungs. The FDA states there is a reasonable probability of serious or life-threatening infections for individuals with weakened immune systems. No adverse events had been reported at the time of the recall notice, but vigilance is advised.
Steps to Take if You Have ReBoost Nasal Spray Lot 224268
- Confirm the lot number. Look on the bottle label and outer carton. Only lot 224268 is impacted.
- Discontinue use immediately. Keep the bottle sealed; do not discard contents down the drain.
- Request a refund or replacement.
- Bought directly from MediNatura? Email recall@medinatura.com for instructions.
- Bought at a retailer? Return the product to the store in accordance with its return policy.
- Report any side effects. Submit complaints to the FDA MedWatch Program via the online form.
- Seek medical attention if needed. Contact a healthcare provider if you experience symptoms of infection (e.g., fever, sinus pain, shortness of breath).
Official Images Included in the Recall Notice

Frequently Asked Questions
- What ReBoost Nasal Spray lot is included in the FDA recall?
- Only ReBoost Nasal Spray 0.68 fl oz (20 mL) bearing lot 224268 and expiration 12/2027.
- Why did MediNatura recall ReBoost Nasal Spray lot 224268?
- Quality testing revealed yeast, mold and a species of Achromobacter bacteria above acceptable limits, creating a potential infection risk.
- How do I verify my ReBoost Nasal Spray lot number?
- Locate lot 224268 on the bottom of the carton or side of the bottle; confirm the NDC 62795-4005-9 and UPC 787647101863.
- What should I do with my recalled ReBoost Nasal Spray?
- Keep the bottle sealed, stop using it immediately, and follow the refund or return steps listed above.
- Are other ReBoost products affected by this recall?
- No. The recall is limited to ReBoost Nasal Spray lot 224268. Always double-check lot numbers before use.
- Has MediNatura recalled ReBoost Nasal Spray before?
- According to FDA records, this is the first recall involving ReBoost Nasal Spray for microbial contamination.